Experiment of preparing long afterglow materials by combustion method
Experiment part:
Objective:
- Learn to use the combustion method to rapidly grow the afterglow materials.
- Understand long afterglow material and its long afterglow principle.
- Learn to rapidly synthesize inorganic oxides and nanomaterials by combustion method.
The synthesis method of long afterglow materials and the related steps of combustion synthesis experiment
Experiment principle:
Combustion is also called self – propagating high – temperature synthesis. It is a kind of chemical system with high exothermic heat induced local chemical ignition through external energy, forming frontier combustion wave, making the chemical reaction continue to spread until the whole system reaction.
This study is through the use of metal nitrate and organic dyes mixed system, via dissolved in solvent to form a solution after mixing, high temperature combustion synthesis of aluminate, metal nitrates in the high temperature boiling, concentration, smoke and fire burning rapidly, flame spread in the form of combustion wave self-sustaining, heaviest foamy powder.
2 al (NO3)3 + Sr(NO3)2 + 20/3co (NH2)2 + 10O2 – SrAl2O4 + 40/3h2o + 32/3n2 + 20/3co2
Experimental equipment and materials:
Main reagents: Eu2O3,Dy2O3, strontium nitrate, aluminum nitrate, urea, boric acid, dilute nitric acid
Equipment and instruments: analytical balance, beaker, glass rod, pipette, equine boiler, jade crucible, fluorescence photometer
Experiment steps:
(I) preparation of precursor: 0.088g Eu2O3, quasi-name; 0.093 grams of Dy2O3; 2.116 g strontium nitrate, 7.5 g aluminum nitrate, 15 urea; 0.18g boric acid.
- The rare earth oxides were dissolved by heating with A certain amount of nitric acid to form the rare earth ion nitrate ion solution A.
- Weigh good strontium nitrate, aluminum nitrate, urea and boric acid, and dissolve them with a certain amount of water. Heat the mixture to about 70 degrees Celsius and obtain solution B.
- In the stirring state, slowly add A to B, keep heating and stirring for A while, make the system mix evenly and evaporate some water.
- Transfer the mixture to the jade crucible, that is, get the precursor that can be used for combustion.
(2), the sample of the combustion synthesis: first the horse boiling preheat oven to 600 degrees Celsius, and then put in the precursors before the fast, can be observed in a few minutes of reactants violent boiling, expansion, with the spread of top-down, take it out of the reaction, cooling product obtains the foamy synthesis — long persistence materials.
Characterization: after the sample is taken out, the sample is loaded, and the emission and absorption spectra of the sample are measured with a spectrophotometer, and the corresponding data are obtained, sorted out, and the relevant atlas is obtained through software fitting.
- Result analysis
Fluorescence excitation spectrum:
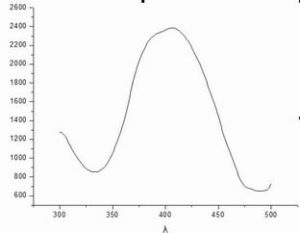
Data and excitation spectrum analysis: the wavelength of excited light corresponding to the place with the highest intensity, that is, the maximum wavelength of excited light is 406.6nm, where the molecular absorption energy is the largest and the number of molecules in the excited state is the largest, thus producing the strongest fluorescence.
Fluorescence emission
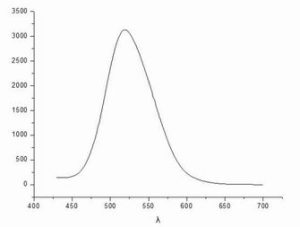
Data and emission spectrum analysis: the wavelength of emission light corresponding to the place with the maximum intensity, that is, the maximum emission wavelength is 518.6nm.
In conclusion, due to the characteristics of fluorescent measuring instruments, such as energy distribution of light source, transmittance of monochromator and sensitivity of detector, the excitation and emission spectra measured under general conditions are all apparent spectra. The apparent spectrum of the same fluorescent compound in different fluorescence instruments is different from each other. Only the corrected spectral shape is consistent.


 中文
中文